Under Construction 
Common Household products chemical composition
PAINTS, STAINS, AND FINISHING PRODUCTS
Because of stricter regulations on Volatile organic compounds (VOCs) more paints are coming out as Latex with improved properties.
See: Paints set a new green standard.
and the painting page here.
- Latex or Water Based paints (glycol esters, phenyl mercuric acetate)
- Rust paints (methylene chloride, tolulene, petroleum distillates)
- Oil-based or Enamel paints (ethylene, aliphatic hydrocarbons)
- Wood preservatives (chlorinated phenols, creosote, copper or zinc napthenate)
- Turpentines and Thinners (n-butylalcohol, acetone, petroleum distillates)
- petroleum distillates
- mineral spirits (paint thinner),
-
- Turpentine is a distillation of pine-tree sap. Before the mid-20th century, turpentine was widely used as a thinner and clean-up solvent for oil paint and varnish, and also as a grease and wax cleaner.
- Stains and Finishes (glycol esters, ketones, naphtha, halogenated hydrocarbons)
- Furniture strippers (acetone, alcohols, xylene, tolulene, methylene chloride)
HOUSEHOLD CLEANERS
- Disinfectants ( lye (NaOH) or historically potassium hydroxide (KOH)., cresol (methylphenols C7H7OH), ammonia (NH3), phenols, diethylene glycol)
- Rug cleaners (naphthalene, oxalic acid, diethylene glycol)
- Toilet bowl cleaners (oxalic acid, calcium hypochlorite)
- Abrasive cleaners (ethanol, trisodiumphosphate, ammonia)
- Oven cleaners (ammonia, potassium or sodium hydroxide)
- Ammonia cleaners (ammonia, ethanol)
- Metal cleaners (acidified thiourea, sulfuric acid)
- Bleach cleaners (sodium or potassium hydroxide, sodium or calcium hypochlorite)
- Drain cleaners (hydrochloric acid, petroleum distillates)
OTHER HOUSEHOLD
- Mineral oil - a transparent, colorless oil composed mainly of alkanes (typically 15 to 40 carbons)[1] and cyclic paraffins (C24).
- Rubbing alcohol - Isopropyl or ethyl alcohol to which some methyl or acetone has been added to make it poisonous.
- Floor/Furniture polish (ammonia, phenol, diethylene glycol)
- Shoe polish (Avoid those containing methyl chloride, trichloroethylene or nitrobenzene)
- Glues (including epoxy, plastic adhesives, rubber cement, instant glue, model glue. These contain toxins)
- Pool chemicals (chlorine, muriatic acid, petroleum distillates, algicides, sodium hypochlorite)
- Ant and roach killers (organophosphates, carbamates, pyrethrins)
- Nail polish and remover (Polish-formaldehyde resin, phenol, tolulene, xylene. Remover--- acetone)
- Houseplant insecticides (methoprene, malathion, tetramethrin and carbaryl)
- Flea and tick repellents (carbamates, pyrethrins and organophosphates)
- Mothballs (naphthalene and paradichlorobenzene)
- Drycleaning solvents
AUTOMOTIVE PRODUCTS
- Windshield wiper fluid (methanol)
- Used motor oil (hydrocarbons like benzene, lead, zinc)
- Antifreeze (ethylene glycol [C2H4(OH)2])
- Car wax (naphtha)
- Gasoline and diesel fuel (tetraethyl lead in leaded form//benzene and ethylene dichloride in unleaded form)
- Transmission fluid (hydrocarbons, mineral oils)
- Batteries for automobiles, boats (sulfuric acid and lead)
- Brake fluid (glycol ethers)
Source: The Green Community - How to Dispose of Chemicals Safely
Chemical Info:
- Alkene
- Have carbon=carbon double bonds.
e.g. Ethylene C2H4,
2-methyl-2-butene (C5H10)
- Ethylene
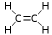 C2H4 The simplest alkene. Because it contains a carbon-carbon double bond, ethylene is called an unsaturated hydrocarbon or an olefin. It is extremely important in industry and also has a role in biology as a hormone. Ethylene is the most produced organic compound in the world.
C2H4 The simplest alkene. Because it contains a carbon-carbon double bond, ethylene is called an unsaturated hydrocarbon or an olefin. It is extremely important in industry and also has a role in biology as a hormone. Ethylene is the most produced organic compound in the world.
It is normally produced in small quantities by most fruits and vegetables. Many fruits produce larger quantities of ethylene and respond with uniform ripening when exposed to an external source of ethylene.
See: Ethylene Facts
- Glycol Ester
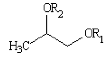 Where R1 and R2 represent one fatty acid moiety and hydrogen in the case of mono-esters and two fatty acid moieties in the case of di-esters.
Where R1 and R2 represent one fatty acid moiety and hydrogen in the case of mono-esters and two fatty acid moieties in the case of di-esters.
Glycol Ester Emulsifiers used in food.
- Methylene chloride or Dichloromethane (DCM)
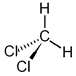 CH2Cl2 CH2Cl2
One of the less harmful of the chlorocarbon solvents.
Links:
Hydrocarbons
Disinfectants
Return to Chemistry
last updated 14 Nov 2008
|
