Links:
Federal Trade Comission (FTC)
About Octane at chemistry.about.com
Why use premium gas when regular will do? at usatoday.
Many people still think that higher octane is better for a variety of reasons including:
- Its higher quality gas and will give better performance or mileage.
- It has more additives to keep your engine cleaner.
In fact, in most cases, using a higher octane gasoline than your owner's manual recommends offers absolutely no benefit. It won't make your car perform better, go faster, get better mileage or run cleaner. It could even be damaging to your car.
The U.S. Environmental Protection Agency requires that all octane grades of all brands of gasoline contain engine cleaning detergent additives to protect against the build-up of harmful levels of engine deposits during the expected life of your car.
What is Octane:
In internal combustion engines, the compressed gasoline-air mixtures have a tendency to ignite prematurely rather than burning smoothly. This creates engine knock, a characteristic rattling or pinging sound in one or more cylinders.
Higher octane fuels reduce the tendency for knock and generally
burn slower.
Higher performance engines use higher compression ratios (the gas and air in the cylinders is more packed more densely) which increase the tendency for pre-ignition and caused the fuel to burn faster because the hydrocarbon molecules are closer together, thus requiring higher octane fuel. Typically a compression ratio of 7.5 requires 85 octane fuel, while a compression ratio of 10.0 requires 100 octane fuel.
Gasoline is made up of a mixture of hydrocarbon molecules and additives. Part of the refining process includes an isomerization process, where straight chain alkanes like pentane are converted into branched chain isomers like 2-methylbutane, which burn more efficiently.
The basic combustion process converts gas (HydroCarbon molecules of Hydrogen and Carbon) and Oxygen to Carbon dioxide (CO2), water vapor (H2O) and energy, heat (58%) and power from expanding gasses (42%).
Some of the power is lost to compress the gas/air mixture prior to combustion and friction so 26% of the energy is left as usable power.
Other chemicals may be produced under certain conditions. See combustion gasses below.
n-Heptane, which is a long string of hydrocarbons [CH3-CH2-CH2-CH2-CH2-CH2-CH3], is assigned an octane number of 0.
isooctane (2,2,4-trimethylpentane), an isomer with more branches
CH3 H
| |
CH3—C—CH2—C—CH3
| |
CH3 CH3
is assigned an octane number of 100.
The aromatic hydrocarbons ("aromatics"), toluene and xylene are octane improvers.
Octane usually ranges from 87 to 93.
The recommended gasoline for most cars is regular - 87 octane.
Premium gas has octane ratings of 91 or higher depending on the state.
In the Rockies where lower octane is recommended because of the thin air (see below) typical ratings are 85, 87, and 91.
What is knocking?
It is the piston rattling around in the engine cylinder and can be the result of:
- The fuel mixture igniting too early so it is pushing against the piston while it is still moving up towards top dead center (TDC).
- fuel-air mixture burning unevenly because it ignites spontaneously on one side of the cylinder.
It can be caused by either bad engine timing (the spark going off too soon) or spontaneous ignition (detonation) from heat and the highly compressed mixture before the spark goes off.
Early detonation results in multiple flame fronts within the combustion chamber instead of a single flame kernel. When these multiple flames collide, they do so with explosive force that produces a sudden rise in cylinder pressure accompanied by a sharp metallic pinging or knocking noise. The hammer-like shock waves created by detonation subject the head gasket, piston, rings, spark plug and rod bearings to severe overloading.
Some Causes are:
- Lean fuel mixture caused by air leaks, dirty fuel injectors, weak fuel pumps or a restricted fuel filter.
- High elevation. (see below)
- Carbon build up in the cylinder which will increase compression and raise temperatures because of an insulating effect. This is more common in older engines which use oil. This can sometimes be removed with a "top cleaner" poured into the carburetor.
- Low boost in a turbocharged engine.
- A spark plug of the wrong heat range.
- Engine running hotter than normal because of ineffective cooling.
Is knocking harmful?
Occasional light knocking or pinging won't harm your engine, and doesn't indicate a need for higher octane. But don't ignore severe knocking. A heavy or persistent knock can lead to engine damage within a week.
What Octane Should I use
Today's engines use highly evolved versions of a device called a knock sensor to adjust timing (firing spark plugs later to adjust for faster burning) automatically for low-octane gas.
Burning regular when the owner's manual specifies premium won't void the warranty, nor damage the engine, even the most finicky automakers say. "You're giving up perhaps just a little bit of performance that a customer wouldn't really even notice, it's so slight," says Bob Furey, chemist and fuels specialist at General Motors.
Some auto engineers estimate that power declines roughly 5% with regular when premium is specified.
When should I NOT use regular gas:
- The only modern engines that should really need premium are those with superchargers, which force-feed fuel into the cylinders too fast for the computers to compensate.
- If you are driving on a road where you need all the power you can get for passing or accelerating out of the way you will probably want to stick to higher octane if your engine recommends it.
- If you have an older (pre-'96) car without a knock sensor and it recommends premium.
- If your engine knocks whey you use it.
When should you use premium instead of regular gas:
- If your engine recommends it and you need the extra power (see above).
- A few car engines may knock or ping - even if you use the recommended octane. If this happens, try switching to the next highest octane grade. In many cases, switching to the mid-grade or premium-grade gasoline will eliminate the knock.
When should use regular instead of premium gas:
Oxygen levels found at an altitude of 5,000 ft. are a full 14% less than levels found at sea-level. So high compression engines are compressing less air and the result is like a normal engine at sea level, so hi performance engines may run better on regular at high elevations.
"Most four- and six-cylinder cars that are designed to run on 87 can run in high altitude with 85,"
If you are driving
See more about the chemistry at chemistry.about.com/cs/howthingswork/
Origin of the 'Higher Octane is Better' Concept
Source: chemistry.about.com
Higher octane gasoline did reduce engine knock in older engines that used carburetors to regulate the air/gas mix. The older engines could not regulate the air/fuel mix going into the engine as efficiently as a computerized fuel injector. A carburetor in need of adjustment could cause too much fuel to be mixed with the air, which meant the gasoline would not burn completely. The excess gas soaked into carbon deposits and caused a premature ignition of the gasoline from the heat of the engine cylinder.
Since the mid-1980s engines use fuel injectors with computers to accurately control the air/fuel mix over all temperature and environment ranges. The accuracy of the fuel injectors and computers is based on using the recommended gasoline for that engine.
High Performance engines are not hi performance because of high octane fuel. They require high octane to compensate for other characteristics of the engine design (see above).
Combustion Gasses:
Gasoline is a complex mixture of all kinds of chemical compounds, but if you add them all up and average them out you come up with the chemical formula for iso-octane (C8H18). This is 8 carbon atoms chemically bonded to 18 hydrogen atoms and that's why they call it a hydrocarbon. The right amount of air to mix with one molecule of isooctane would be 12.5 oxygen molecules (O2) and 47 nitrogen molecules (N2). The combustion process would result in 8 carbon dioxide molecules (CO2), 9 water molecules (H20) and 47 nitrogen molecules (N2). So, the chemical formula for "perfect" gasoline combustion is:
C8H18 + 12.5 O2 + 47 N2 —> 8 CO2 + 9 H2O + 47 N2
The Nitrogen in the air should be unchanged.
If an engine is running too rich (not enough O2 or too much fuel it will produce carbon monoxide (CO) instead of CO2
If nitrogen is heated above 2500 degrees (Fahrenheit) it will burn or oxidize just like gasoline combines with oxygen in our equation. The resulting NOX can be nitric oxide (NO), nitrous oxide (N20) or nitrogen dioxide (NO2) or one of several others, none of which are very stable chemicals. Most tailpipe NOX is nitrogen dioxide.
Small amounts of Sulfur dioxide (SO2) from some sulphur in the gas.
During certain conditions the catalyst oxidizes SO2 to SO3 which reacts with water to make sulfuric acid (H2SO4)
Nitrous Oxides (NOx) are produced from excessive combustion temperatures.
Lean mixtures lead to hot combustion with extra oxygen so just as CO is a good rich indicator NOX is a good lean indicator up until the fire goes out.
What we want to see coming out the tailpipe is low CO, NOx, HC and oxygen with high carbon dioxide and lots of water vapor.
Catalytic Converter:
Most modern cars are equipped with three-way catalytic converters. "Three-way" refers to the three regulated emissions it helps to reduce -- carbon monoxide, VOCs and NOx molecules.
The reduction catalyst is the first stage of the catalytic converter. It uses platinum and rhodium to help reduce the NOx emissions. When an NO or NO2 molecule contacts the catalyst, the catalyst rips the nitrogen atom out of the molecule and holds on to it, freeing the oxygen in the form of O2. The nitrogen atoms bond with other nitrogen atoms that are also stuck to the catalyst, forming N2.
The oxidation catalyst is the second stage of the catalytic converter. It reduces the unburned hydrocarbons (VOCs) and carbon monoxide by burning (oxidizing) them over a platinum and palladium catalyst. This catalyst aids the reaction of the CO and hydrocarbons with the remaining oxygen in the exhaust gas.
The third stage is a control system that monitors the exhaust stream, and uses this information to control the fuel injection system. There is an oxygen sensor mounted upstream of the catalytic converter, meaning it is closer to the engine than the converter is. This sensor tells the engine computer how much oxygen is in the exhaust. The engine computer can increase or decrease the amount of oxygen in the exhaust by adjusting the air-to-fuel ratio.
Source: How Stuff Works
Seasonal Differences:
During the summer, pollution is a frequent concern due to increased levels of smog and ozone.
RVP is the vapor pressure of gasoline measured at 100 degrees Fahrenheit. Fuels with higher RVP evaporate more easily than those with lower RVP.
Depending on the part of the country, the EPA's standards mandate an RVP below 9.0 PSI or 7.8 PSI for summer-grade fuel. Some local regulations call for stricter standards.
Because RVP standards are higher during the winter, winter-grade fuel uses more butane, with its high RVP of 52 PSI, as an additive.
Ethanol:
Ethanol is made in U.S. from plant material, such as corn, sugar cane, or grasses.
E10 and E15 are blends of ethanol and gasoline—the number after the "E" indicates the percentage of ethanol by volume.
See:
Summer-grade versus Winter-grade Fuel - HowStuffWorks
Ethanol | fueleconomy.gov
Glossary:
- Air/Fuel Ratio
- The ratio of pounds of Air to pounds of Fuel needed for combustion in an engine. A/F Ratios range from about 2:1 for NitroMethane to about 16:1 for gasoline, with 14.7:1 considered the stoichiometric or chemically correct ratio under perfect conditions with normal (non-oxygenated) gasoline. Gasoline A/F ratios for best power tend to be in the 13.25:1 - 13.75:1 range.
- Alternative Fuels
- Alternative fuels, as defined by the Energy Policy Act of 1992 (EPAct), include ethanol, natural gas, propane, hydrogen, biodiesel*, electricity, methanol, and p-series fuels.
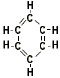
- Aromatic
| High octane blending components that have a benzene ring in their
molecular structure (benzene, toluene, xylene). |
 |
- Detergent
- Additive used to prevent and/or clean up carburetor and fuel injector
deposits.
- Ethanol - (EtOH)
- Ethanol is an alcohol-based alternative fuel produced by fermenting and distilling starch crops that have been converted into simple sugars. Feedstocks for this fuel include corn, barley, and wheat. Ethanol can also be produced from "cellulosic biomass" such as trees and grasses and is called bioethanol. Ethanol is most commonly used to increase octane and improve the emissions quality of gasoline.
- Gasohol
- In the U.S., the term gasohol refers to gasoline which contains 10%
ethanol. This term was used in the late 1970's and early 1980's but has been
replaced by terms such as Super Unleaded Plus Ethanol or Unleaded Plus.
- KS - Knock Sensor
- Device on the engine that responds to the frequency vibrations characteristically produced by detonation (typically 6-8kHz). The knock sensor produces a voltage signal that signals the computer to momentarily retard ignition timing until the detonation stops.
A knock sensor can usually be tested by rapping a wrench on the manifold near the sensor (never hit the sensor itself!) and watching for the timing change while the engine is idling.
- Methanol - (MeOH)
- Methanol, also known as wood alcohol, can be used as an alternative fuel in flexible fuel vehicles that run on M85 (a blend of 85% methanol and 15% gasoline). However, it is not commonly used because automakers are no longer supplying methanol-powered vehicles.
In the future, methanol could possibly be the fuel of choice for providing the hydrogen necessary to power fuel cell vehicles.
- MON Motor Octane Number
- Determined with a test engine running at 900 rpm.
- MTBE - Methyl Tertiary Butyl Ether
- Oxygen bearing chemical (ether) that is added to fuel to bring additional oxygen to the combustion process. MTBE production and use has declined because it has been found to contaminate ground water.
- NOx
- Nitrogen oxides (NO and NO2, together called NOx) - contributes to smog and acid rain, and also causes irritation to human mucus membranes
- Octane Number
- A measure of the anti-knock characteristics of a given fuel. in the US equal to (RON+MON) /2 .
- RON Research Octane Number
- Determined with a test engine running at a low speed of 600 rpm.
- VOC volatile organic compounds
- Unburned fuel that evaporates.
Glossaries at:
nwicc.com
Fuel Terminology at eric-gorr.com
See Also:
Hydrocarbon Fuels
All About Engine Knock by Brooks Moses
How Engines Make Power
Detonation and pre-ignition at MisterFixIt.com
Alternative Fuels Data Center at the DOE
Exhaust Gas Analysis
An Introduction to Organic Chemistry
last updated 22 Aug 2005
|  Home & Garden
Home & Garden
 Cars
Cars

 Home & Garden
Home & Garden
 Cars
Cars
